Which Ions Are In The Greatest Concentration Outside The Animal Cell
Chapter 22. Osmotic Regulation and Excretion
22.1. Osmoregulation and Osmotic Balance
shrink due to water lossLearning Objectives
By the finish of this department, yous will be able to:
- Define osmosis and explain its role within molecules
- Explain why osmoregulation and osmotic rest are important torso functions
- Depict active send mechanisms
- Explain osmolarity and the way in which information technology is measured
- Draw osmoregulators or osmoconformers and how these tools allow animals to adapt to different environments
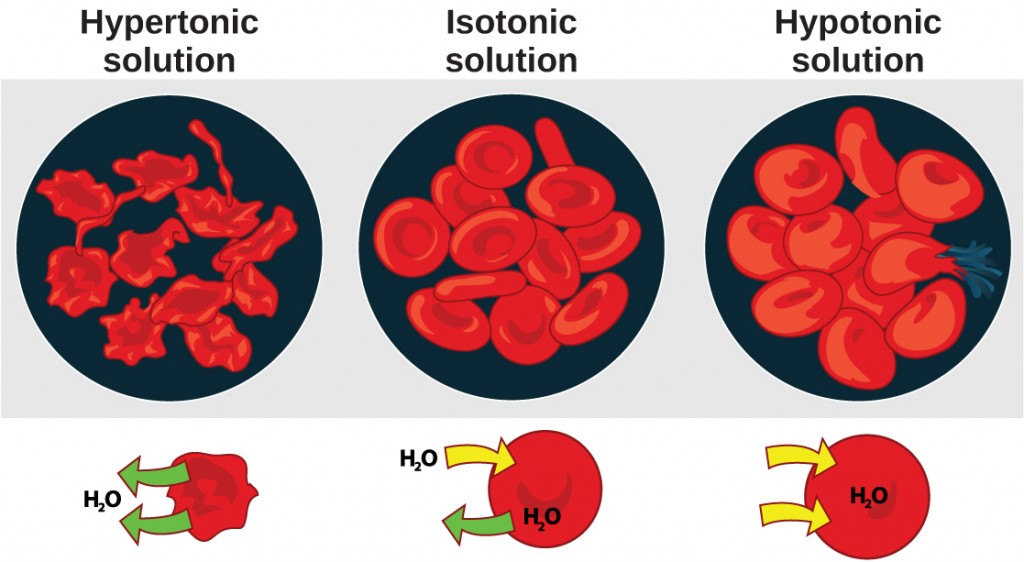
Osmosis is the diffusion of water beyond a membrane in response to osmotic force per unit area caused past an imbalance of molecules on either side of the membrane. Osmoregulation is the procedure of maintenance of salt and water balance ( osmotic residue) beyond membranes within the trunk's fluids, which are equanimous of h2o, plus electrolytes and non-electrolytes. An electrolyte is a solute that dissociates into ions when dissolved in water. A non-electrolyte, in dissimilarity, doesn't dissociate into ions during water dissolution. Both electrolytes and non-electrolytes contribute to the osmotic balance. The body'southward fluids include blood plasma, the cytosol within cells, and interstitial fluid, the fluid that exists in the spaces between cells and tissues of the body. The membranes of the body (such equally the pleural, serous, and cell membranes) are semi-permeable membranes. Semi-permeable membranes are permeable (or permissive) to certain types of solutes and water. Solutions on two sides of a semi-permeable membrane tend to equalize in solute concentration by motion of solutes and/or h2o across the membrane. As seen in Figure 22.two, a cell placed in water tends to swell due to proceeds of h2o from the hypotonic or "low salt" environment. A prison cell placed in a solution with higher common salt concentration, on the other hand, tends to make the membrane shrivel up due to loss of water into the hypertonic or "high salt" surroundings. Isotonic cells have an equal concentration of solutes inside and outside the cell; this equalizes the osmotic pressure on either side of the jail cell membrane which is a semi-permeable membrane.
The body does not exist in isolation. At that place is a constant input of water and electrolytes into the system. While osmoregulation is accomplished across membranes inside the body, excess electrolytes and wastes are transported to the kidneys and excreted, helping to maintain osmotic residuum.
Need for Osmoregulation
Biological systems constantly collaborate and exchange water and nutrients with the environs past way of consumption of food and water and through excretion in the form of sweat, urine, and feces. Without a mechanism to regulate osmotic force per unit area, or when a disease damages this mechanism, there is a tendency to accumulate toxic waste and water, which tin can have dire consequences.
Mammalian systems have evolved to regulate non simply the overall osmotic force per unit area beyond membranes, but also specific concentrations of important electrolytes in the three major fluid compartments: blood plasma, extracellular fluid, and intracellular fluid. Since osmotic pressure level is regulated by the movement of water across membranes, the volume of the fluid compartments can also change temporarily. Considering blood plasma is i of the fluid components, osmotic pressures take a direct bearing on blood pressure.
Transport of Electrolytes across Cell Membranes
Electrolytes, such as sodium chloride, ionize in water, meaning that they dissociate into their component ions. In water, sodium chloride (NaCl), dissociates into the sodium ion (Na+) and the chloride ion (Cl–). The most important ions, whose concentrations are very closely regulated in body fluids, are the cations sodium (Na+), potassium (K+), calcium (Ca+2),
magnesium (Mg+2), and the anions chloride (Cl–), carbonate (CO3 -2), bicarbonate (HCO3 –), and phosphate(PO3 –). Electrolytes are lost from the body during urination and perspiration. For this reason, athletes are encouraged to supersede electrolytes and fluids during periods of increased activity and perspiration.
Osmotic pressure is influenced by the concentration of solutes in a solution. It is directly proportional to
the number of solute atoms or molecules and not dependent on the size of the solute molecules. Because electrolytes dissociate into their component ions, they, in essence, add more solute particles into the solution and have a greater effect on osmotic pressure, per mass than compounds that do not dissociate in water, such as glucose.
Water tin can pass through membranes by passive improvidence. If electrolyte ions could passively diffuse beyond membranes, information technology would exist incommunicable to maintain specific concentrations of ions in each fluid compartment therefore they require special mechanisms to cross the semi-permeable membranes in the torso. This movement tin exist accomplished by facilitated diffusion and active transport. Facilitated diffusion requires poly peptide-based channels for moving the solute. Active transport requires energy in the course of ATP conversion, carrier proteins, or pumps in order to movement ions against the concentration gradient.
Concept of Osmolality and Milliequivalent
In social club to calculate osmotic pressure, it is necessary to sympathize how solute concentrations are measured. The unit for measuring solutes is the mole. One mole is defined as the gram molecular weight of the solute. For example, the molecular weight of sodium chloride is 58.44. Thus, one mole of sodium chloride weighs 58.44 grams. The molarity of a solution is the number of moles of solute per liter of solution. The molality of a solution is the number of moles of solute per kilogram of solvent. If the solvent is water, one kilogram of water is equal to one liter of water. While molarity and molality are used to express the concentration of solutions, electrolyte concentrations are ordinarily expressed in terms of milliequivalents per liter (mEq/Fifty): the mEq/L is equal to the ion concentration (in millimoles) multiplied by the number of electric charges on the ion. The unit of milliequivalent takes into consideration the ions nowadays in the solution (since electrolytes grade ions in aqueous solutions) and the charge on the ions.
Thus, for ions that have a charge of one, one milliequivalent is equal to one millimole. For ions that have a charge of two (like calcium), one milliequivalent is equal to 0.5 millimoles. Another unit for the expression of electrolyte concentration is the milliosmole (mOsm), which is the number of milliequivalents of solute per kilogram of solvent. Trunk fluids are usually maintained within the range of 280 to 300 mOsm.
Osmoregulators and Osmoconformers
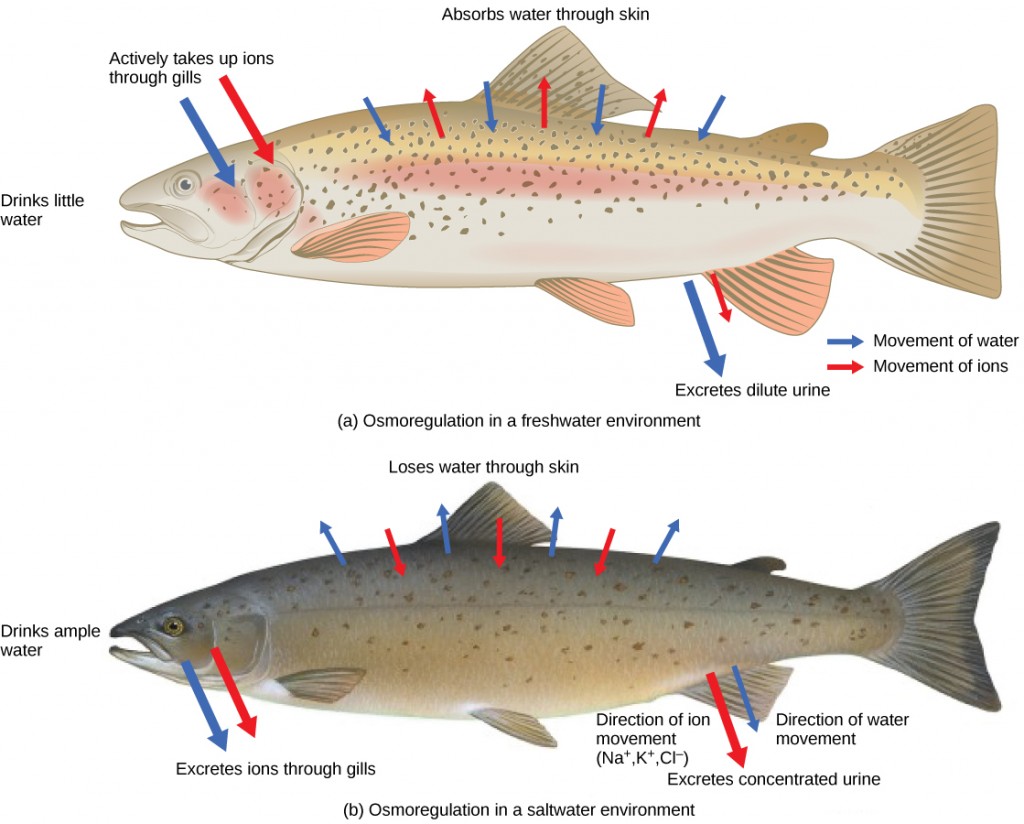
Persons lost at sea without whatsoever fresh water to drinkable are at chance of severe dehydration because the human body cannot accommodate to drinking seawater, which is hypertonic in comparison to body fluids. Organisms such as goldfish that can tolerate only a relatively narrow range of salinity are referred to as stenohaline. About ninety percent of all bony fish are restricted to either freshwater or seawater. They are incapable of osmotic regulation in the opposite environment. It is possible, yet, for a few fishes similar salmon to spend function of their life in fresh water and part in body of water water. Organisms like the salmon and molly that tin tolerate a relatively broad range of salinity are referred to as euryhaline organisms. This is possible because some fish accept evolved osmoregulatory mechanisms to survive in all kinds of aquatic environments. When they live in fresh water, their bodies tend to accept up h2o because the surround is relatively hypotonic, as illustrated in Figure 22.3 a . In such hypotonic environments, these fish exercise not drinkable much water. Instead, they laissez passer a lot of very dilute urine, and they achieve electrolyte residual by active transport of salts through the gills. When they motion to a hypertonic marine environment, these fish start drinking sea h2o; they excrete the backlog salts through their gills and their urine, as illustrated in Figure 22.3 b . Nearly marine invertebrates, on the other hand, may be isotonic with sea water ( osmoconformers). Their body fluid concentrations adjust to changes in seawater concentration. Cartilaginous fishes' salt limerick of the blood is like to bony fishes; however, the claret of sharks contains the organic compounds urea and trimethylamine oxide (TMAO). This does non hateful that their electrolyte limerick is similar to that of body of water water. They achieve isotonicity with the sea by storing big concentrations of urea. These animals that secrete urea are called ureotelic animals. TMAO stabilizes proteins in the presence of high urea levels, preventing the disruption of peptide bonds that would occur in other animals exposed to similar levels of urea. Sharks are cartilaginous fish with a rectal gland to secrete salt and help in osmoregulation.
Dialysis Technician
Dialysis is a medical process of removing wastes and backlog h2o from the blood by improvidence and ultrafiltration. When kidney part fails, dialysis must be done to artificially rid the body of wastes. This is a vital process to keep patients alive. In some cases, the patients undergo artificial dialysis until they are eligible for a kidney transplant. In others who are non candidates for kidney transplants, dialysis is a life-long necessity.
Dialysis technicians typically work in hospitals and clinics. While some roles in this field include equipment evolution and maintenance, most dialysis technicians work in directly patient care. Their on-the-job duties, which typically occur under the direct supervision of a registered nurse, focus on providing dialysis treatments. This can include reviewing patient history and electric current condition, assessing and responding to patient needs before and during handling, and monitoring the dialysis process. Treatment may include taking and reporting a patient's vital signs and preparing solutions and equipment to ensure accurate and sterile procedures.
Summary
Solute concentrations beyond a semi-permeable membranes influence the movement of water and solutes across the membrane. Information technology is the number of solute molecules and non the molecular size that is important in osmosis. Osmoregulation and osmotic balance are of import actual functions, resulting in water and common salt balance. Not all solutes can laissez passer through a semi-permeable membrane. Osmosis is the motility of water across the membrane. Osmosis occurs to equalize the number of solute molecules across a semi-permeable membrane by the movement of water to the side of higher solute concentration. Facilitated diffusion utilizes poly peptide channels to move solute molecules from areas of college to lower concentration while active send mechanisms are required to move solutes against concentration gradients. Osmolarity is measured in units of milliequivalents or milliosmoles, both of which take into consideration the number of solute particles and the accuse on them. Fish that alive in fresh water or saltwater adapt past being osmoregulators or osmoconformers.
Exercises
- When a dehydrated man patient needs to be given fluids intravenously, he or she is given:
- water, which is hypotonic with respect to body fluids
- saline at a concentration that is isotonic with respect to body fluids
- glucose because it is a non-electrolyte
- claret
- The sodium ion is at the highest concentration in:
- intracellular fluid
- extracellular fluid
- blood plasma
- none of the above
- Cells in a hypertonic solution tend to:
- compress due to h2o loss
- swell due to water gain
- stay the same size due to water moving into and out of the cell at the same rate
- none of the above
- Why is excretion of import in gild to achieve osmotic residue?
- Why practice electrolyte ions move across membranes by active transport?
Answers
- B
- B
- A
- Excretion allows an organism to rid itself of waste matter molecules that could be toxic if immune to accrue. Information technology also allows the organism to keep the corporeality of water and dissolved solutes in balance.
- Electrolyte ions often crave special mechanisms to cross the semi-permeable membranes in the body. Agile send is the movement confronting a concentration gradient.
Glossary
- electrolyte
- solute that breaks down into ions when dissolved in water
- molality
- number of moles of solute per kilogram of solvent
- non-electrolyte
- solute that does non intermission downward into ions when dissolved in water
- molarity
- number of moles of solute per liter of solution
- mole
- gram equivalent of the molecular weight of a substance
- osmoconformer
- organism that changes its tonicity based on its environs
- osmoregulation
- mechanism by which water and solute concentrations are maintained at desired levels
- osmoregulator
- organism that maintains its tonicity irrespective of its environment
- osmotic residuum
- balance of the amount of water and salt input and output to and from a biological arrangement without agonizing the desired osmotic pressure and solute concentration in every compartment
- osmotic pressure
- pressure exerted on a membrane to equalize solute concentration on either side
- semi-permeable membrane
- membrane that allows only sure solutes to pass through
Source: https://opentextbc.ca/biology/chapter/22-1-osmoregulation-and-osmotic-balance/
Posted by: reyesaffir1968.blogspot.com

0 Response to "Which Ions Are In The Greatest Concentration Outside The Animal Cell"
Post a Comment